No products in the cart.
NZDA News Article, Infection Prevention and Control
Make it Mechanical!
The IPC equivalent of digital dentistry is… manual vs mechanical instrument reprocessing. What is the best direction to take for your reprocessing workflow and sterilisation room?
That was then—this is now. The old ‘steri bay’, far from sight of any patients, never to see horrors of used dental instruments. This is no longer the case. The image of sterilisation areas has changed dramatically. Once hidden away, now often more open, and sometimes an impressive focal point of many practices!
That was then… this is now
Patients have become more aware of IPC best practices and are actively looking to see appropriate steps, or they are simply trusting you to do it! Your reprocessing area can send a message that you care about the environment you treat them in. When placed in a high traffic location it offers transparency and builds confidence in staff and the practice.
The advantages of a mechanical reprocessing workflow are many, and every practice is likely to benefit. Not only in terms of compliance, but also with enhanced efficiency and health and safety.
The need for change—automated reprocessing vs manual process for cleaning instruments, the case is strong. A common question is will the use of washer disinfectors in dental practices become mandatory? The answer is ‘likely yes’. The only debate is around the timing. Ever increasing knowledge regarding disease transmission and the need to safeguard patients makes it inevitable in all deliveries of healthcare, including dentistry. Thirty years ago gloves were not worn routinely, and are now an accepted part of all treatment—imagine patient reaction if not!
The ‘Big Picture’—‘The complete, overarching story or idea. Implies that one should think of the future, or think of other parallel factors, and not focus on small details’. There are many advantages for a mechanical approach other than in terms of compliance; enhanced efficiency and health and safety—every practice is likely to benefit.

Out with the old, in with the new. Traditionally, dental instruments have been cleaned by either manual scrubbing and mechanical cleaning, often both. However, routine hand scrubbing of dental instruments is not recommended and should be discontinued.
The most common method for cleaning dental instruments is manual washing, usually with, but sometimes without the use of an ultrasonic bath. However, the manual wash process is poorly controlled, many practices use no cleaning agent other than water. Not all practices use a detergent specifically formulated for manual washing of instruments, and the time between changes of ultrasonic bath cleaning solution can range from two to eight hours (it should be event related not time bound).
Most practices have a dedicated area for instrument reprocessing, unfortunately some are within the patient treatment area. Clear delineation of the clean and dirty areas still remains an issue.
Nearly all reprocessing of dental instruments is undertaken by dental assistants, with training provided mainly by demonstration and observed practice of a colleague. Comprehensive synchronous training that takes place in real-time with an instructor, allowing the learner to engage with both the trainer and with others performing the tasks is much preferred for this type of skill. Appropriate documentation of methodology, skills shown, observed, and demonstrated all outlined clearly in a Training Schedule, and once signed documented in a Training Register as part of the practice Policy & Procedure Manual. Remember, if it’s not documented it’s not considered done.
Results from a significant number of practice audits indicate; most staff wore gloves when undertaking manual cleaning, but the gloves worn are procedural and not suitable for handling used instruments or chemicals, 51% of staff don’t use eye protection, 57% don’t wear a mask!
In many dental practices, the cleaning of re-usable dental instruments is undertaken using poorly controlled processes and procedures, increasing the risk of cross infection. Clear and unambiguous advice must be provided to the dental team regarding appropriate equipment, chemicals and the environment for cleaning dental instruments. This should be facilitated by appropriate training and the implementation of quality assurance procedures at each stage of the cleaning process.
No, no, no! Stop that! Routine hand scrubbing of dental instruments is not recommended.
Many practices use manual cleaning as either; the only process, or part of process (before ultrasonic).
The concerns and issues of a manual only process are very clear:
- High risk
- Aerosols
- Biofilm & bioburden
Deciding on cleaning methods—considerations;
- Most effective
- Compatible with items
- Occupational health and exposure risks to staff
The main considerations in selecting appropriate cleaning methods include effectiveness, compatibility with the items that require cleaning and the occupational health and exposure risks posed to staff with each method.
Manual cleaning should only be undertaken when the manufacturer specifies a device is either incompatible with automation processes, or when the washer disinfector is temporarily unavailable—for example, the unit is being repaired or validated.
If/when manual cleaning will be included there must be a written procedure to be followed and comprehensive training provided. This should support ability to control critical parameters as far as possible to reduce variability in cleaning performance.
Ensure a low-foaming detergent specifically formulated for cleaning instruments is used, mixed exactly to manufacturer’s instructions, water temperature should not be over 45˚C (above protein coagulation point), with items fully immersed to minimise splashing and minimise aerosol risk.
Use of metal bristled instrument brushes is also not recommended as they can be extremely damaging to the instrument surfaces, and/or particularly to diamond coated burs. Use of an instrument foam (magic if you haven’t used it yet!) instead is recommended. Add it to the list—it’s a game changer (time and efficiency).
‘Best practice’—or not? All in all, manual cleaning not considered ‘best practice’ as it is difficult to validate due to variables and inconsistencies in time availability and individual cleaning styles, and ultimately it carries a greater risk of injury to staff compared with mechanical methods.
Manual cleaning/washing summary—what’s the issue?
- Often no designated ‘instrument cleaning only’ sink used; hand washing, beverage preparation, environmental cleaning sinks are also used.
- Poorly controlled—no cleaning agent / water only.
- Range of cleaning agents used; surgical hand wash, flowing or bar soap, disinfectants, dishwashing liquid, incorrect concentration and/or water temperature. Note—clinical detergent formulated for instrument management must be used.
- Range of brush types used—more than one type; synthetic, wire or natural bristles, pipe cleaners, metal/nylon pot scourers (ouch for the instrument!)
- Cleaning lumened devices (HVE) using brushes on wire stem or pipe cleaner.
Show me some numbers… many practices use manual washing as either the only method, or as part of the cleaning process. 43% have a designated sink which is used only for instrument cleaning, but many also use the same sink for hand washing (84%), beverage preparation (16%), or environmental cleaning (34%). The manual washing process is generally poorly controlled, 41% of practices using only water, without cleaning agent. Only 2% of practices use a detergent formulated for manual washing of surgical instruments, 37% using surgical hand wash. Other miscellaneous agents used for cleaning include bar soap, dishwashing liquids and disinfectants. A range of cleaning agents are used without standardisation of concentration or of the temperature of water used for cleaning.
The range of brush types used to clean instruments included synthetic bristles (70%), wire bristles (46%), natural bristles (9%) or pipe cleaners (8%), many use more than one type of brush. Other items used to clean instruments include metal pot scourers (4%) and nylon pot scourers (3%). Cleaning of lumened devices, such as suction tips, 64% use wire stem brushes, or 89% a pipe cleaner. Only 14% performed manual cleaning with instruments entirely immersed to prevent aerosol generation, and 60% perform manual washing entirely under running water. Rinsing of washed instruments was undertaken in 84% of practices, but not often in hot water. Only 10% of surgeries used a separate sink for rinsing and a further 2% rinsed in a bowl. Drying of instruments (never leave to air dry) after manual cleaning occurs in around 85% of practices.
Touchless reprocessing workflow

and time is also precious!
Efficient reprocessing workflow provides both cost and time saving opportunities—is yours maximised? Is your DA available for the important tasks; patient care, clinical support chairside, operatory changeover? Or are they spending valuable time ‘doing the dishes’?
In the words of DA’s …
“I spend so much time sorting instruments & packaging”
“My time would be better spent with patients”
“Minutes matter when you’re on an already tight schedule, or have an emergency patient coming”
Reprocessing workflow
- Efficient instrument reprocessing
- Highest protection for patients & clinical team
Defined as ‘a physical or chemical procedure to decontaminate re-usable medical devices for further use on patients and handling by staff’. The aim of which is to protect patients and the practice staff from harmful microorganisms on the instruments.
The complete instrument decontamination process consists of; cleaning and disinfection, inspection, packaging, sterilisation, including parametric release, batch control identification (BCI) and documentation of process (linking patient to process).
All guidelines, including international, all state the entire process of instrument reprocessing needs to be performed by trained personnel using monitored procedures to ensure reproducibility.
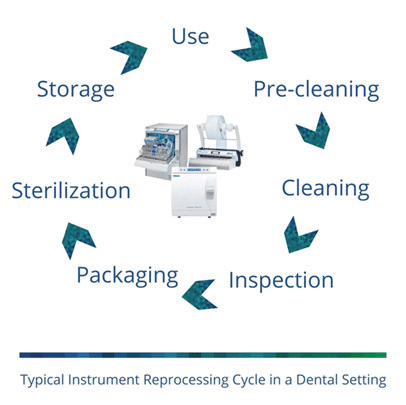
What is the instrument reprocessing cycle? Automated equipment is most efficient method available and can increase productivity—an important and measurable component.
One Way Workflow—assess, review and realign as required. Apply critical thinking of the how and why of IPC evidenced based decision-making processes.
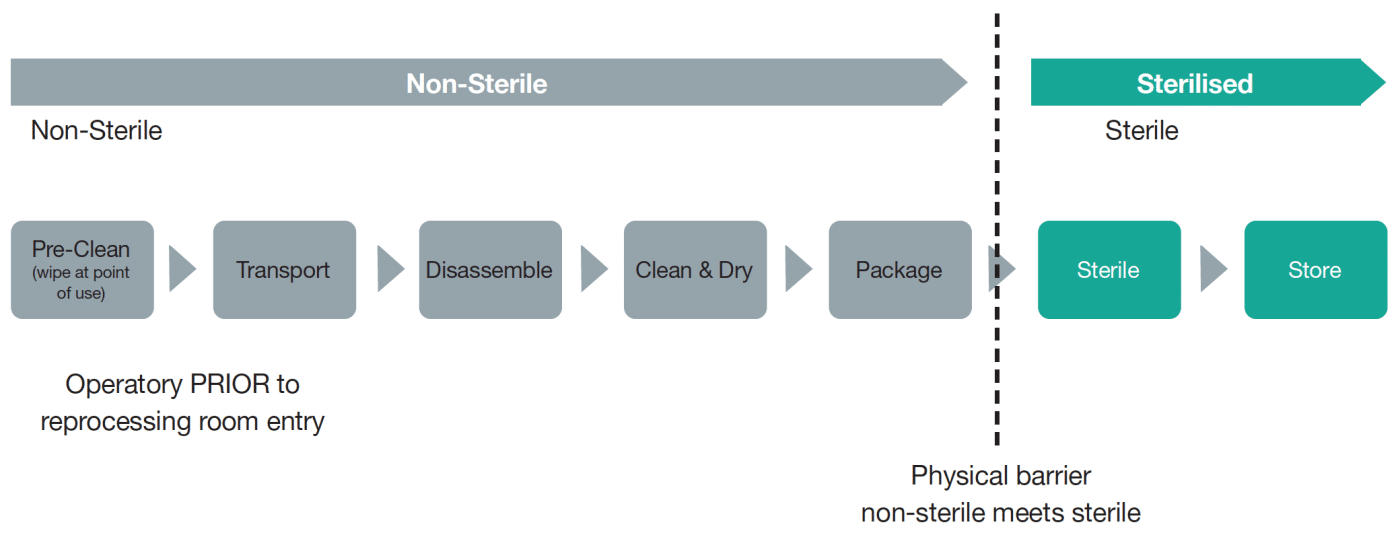
Ensure workflow is one direction—dirtiest to cleanest. Each ‘level’ incrementally reduces microbial load on devices being reprocessed (including cleaning, disinfection and sterilisation) and prevents contamination of items processed to higher level having contact with lower level processed medical device or areas.
Considerations include: dirty-to-clean process workflow, touchless sinks or drawers and durable finishes to withstand high moisture levels. Each area (or ‘station’) is dependent on another. Operating in anything but a one-way workflow poses an IPC risk. Defining the details of each station is important to connect the station and understand how each serves a specific role and why that role is needed to keep moving in the one-way direction.
Review and assess your one-way workflow. Do you have any enhancements to make? Are you already performing with a high functioning one-way workflow?
‘We don’t know what we don’t know’—true! However, once we know then it is important to act accordingly, make any necessary changes and set goals as applicable. These moments of reflection are good examples of exemplary professional growth.
Clean before you steam / Mechanical NOT manual!
Ultrasonic cleaning—ultrasonic energy passed through a solution to ‘shake’ debris loose. Correct ultrasonic solution formulated for efficient ultrasonic cleaning breaks down bioburden and contains agents to prevent mineral buildup, spotting, and corrosion.
Ultrasonic instrument cleaning effectiveness is based on cavitation where sonic waves generate minute bubbles on instrument surfaces that expand and become unstable, then collapse or implode. The implosion generates a localised vacuum area that literally dislodges/sucks off bioburden—maximised when there is a defined loading pattern and defined maximum load, not when overloaded.
It is critical to use correct solution, mixed according to manufacturer’s instructions and change solution when visibly soiled, and emptied at the end of working day as many practices do. However, solution change interval can range from two to nine hours, not four hourly intervals or more frequently. Occasionally the solution may be changed at the end of working week.
The unit must be regularly cleaned, maintained and performance tested daily. 92% of practices have ultrasonic units that commonly range in age from new/1 year to 10(ish) years, median of three years old. Most (96%) ultrasonic cleaners have removable lids and most (92%) are operated with the lid closed.
After ultrasonic cleaning, 86% of practices rinse instruments, usually under running tap water with variable water temperatures (should be hot). Note—quality of instrument rinse water is an area of impending change.
Automated instrument washer—saves time and eliminates the need to manually rinse or dry instruments. Also protects instruments and therefore provides increased longevity.
Manual scrubbing—should be considered a ‘fall-back’ practice and is no longer/not recommended by regulatory bodies. A manual process takes the most time and effort and carries the highest risk of sharps incidents.
Don’t confuse throughput with productivity
Throughput = number of items passing through the process
Productivity = effectiveness of productive effort
Use of a washer disinfector is currently considered ‘Best Practice’.
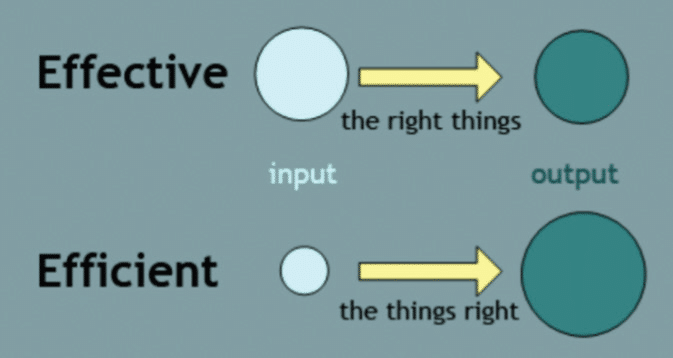
Effective vs Efficient. Process Excellence—what is effectiveness vs efficiency? The key to improving both efficiency and effectiveness is spending the right time, with the right energy, working on the right things, in the right way.
Efficiency is about the ‘means’ while effectiveness is about the ‘end’ result. Efficiency is a metric for speed and cost, while effectiveness is metric for quality and goodness. Critical differential.
Asking, “Is it effective?” is really asking if you are achieving the goal. On the other hand, asking, “Is it efficient?” is asking if you are doing it at the least cost in terms of time, money, and energy.
Efficiency is doing things right; effectiveness is doing the right thing. As Sheldon would say—bazzinga!
Considerations for efficiency and effectiveness for use of washer disinfector (WD):
- Saves time – no manual cleaning, no tedious process of drying
- Uses HEPA filter to ensure no additional bacteria or microorganisms transmit onto items during drying process.
Crunch the numbers in your practice…
Time saved approx. 6 minutes per patient tray x 10 pts = 60 minutes per DA.
E.g. $28 hourly rate x 5 days = $140 per DA / 5 x DA’s = $700 per week.
Straight to the point—Do know your total processing time? Knowing your total processing time from dirty to clean can help determine instrument stock levels. Factor return on investment required to increase inventory of certain sets. Conversely, if an instrument has a normal life span of a year, and you now have two, it has increased to two years.
Key to improving efficiency & effectiveness—spending the right time, with the right energy, working on the right things, in the right way.
- Reproducibility
- Consistency
- Quality control
- Validate able
WD Injection rails—used to clean inside of hollowed instruments. Important to check rail has its own filter to ensure your hand pieces don’t become the filter! Handpieces that are mechanically washed with dedicated injection rail last longer when they have been properly cleaned inside. And they do get dirty! Ask a handpiece service technician.
Sharps injuries hurt—yes they do! Use engineering controls to manage, implement safe work practices with routine use of a managed instrument system (cassettes, also referred to as ‘washer trays’ by some manufacturers):
- Patient Preview & Presentation – instruments are organised to procedure type
- Processed & Preparation – instruments kept together throughout reprocessing
- Packaged & Ready – packaged and sterilised together to reduce handling
- Neat & Complete – use or store until required
An organised instrument management system saves time when instruments are organised by procedure and set-up remains intact throughout entire reprocessing; cleaning to sterilisation to storage. The cassette/washing tray is placed directly into ultrasonic or WD and steriliser. Manual handling and scrubbing is eliminated and the risk of potential sharps injuries reduced—‘Touchless Reprocessing’.
The use of a WD removes all variables from the cleaning process. It enables a fully audited and validated process for regulatory compliance. Remove the variables = reproducibility = validation.
Choose carefully—a WD that has the capability to meet practice specific needs regarding space and capacity is important from the outset. For example, one with customisable storage trays that can accommodate a reasonable number of instruments and can achieve a fast turnaround is essential in a busy practice. Choosing a machine with multiple programmes including a ‘fast wash’ is important in terms of maintaining the desired goal of overall efficiency.
An integrated data logger should also be used as it automatically records all the necessary data required for compliance documentation. This removes the need to keep manual records.
Instruments cleaned with automated equipment do not normally need presoaking unless considerable time lapse between used instruments entering the reprocessing cycle—when pre-treatment foam is useful.
What to look for—know your numbers and ask the questions!
- Cycles, accessories, number of patient instruments.
- External dimensions—is it compact? Size matters!
- Active drying—protects instruments from re-contamination, corrosion and damage.
- Batch documentation—with CF card or network?
- Feeding process agents with dosing unit—external or internal? (space saving important)
- How much chemical is being dosed?
Some use only pressure switches to check if chemical is moving but don’t measure exact quantity. It could be a little or a lot, and when dealing in mls this can be a big difference. - Conductivity sensors that monitor incoming water AND wash liquid throughout the cycle?
- How easy is it to swap the chemicals, how often, how much (quantity and cost)?
- Do you have to decant or swap out bottles?—good to know for safety reasons.
- Are the chemicals stored within the unit or do they go in a cupboard externally?
- Maintenance cycles—how many months or cycles? (24, 36 months, or 1000 + cycles?)
- Practice-optimised washing chamber. Customised ‘furniture’ set up—look for numerous baskets and accessories, allow loading configuration for your specific workflow.
- Reprocess instruments for up to how many patients quickly & economically in one cycle?
- Automatic monitoring for rotation of rinse arms, rinse pressure and filter sieves?
- Errors in program mode or during operation prevented with consistent monitoring?—important consideration.
- Floor unit—ergonomic working height & any additional storage space?
- Injector rails—with or without?
Consider HVE tubes or handpieces and contra angles. - Are wash arms monitored for rotation speed, water pressure to ensure correct coverage and avoid spray shadows. Some washers only monitor that the arms are spinning—a simple yes or no, but not actually a check on how well they are spinning.
- Sensors to detect for any water leaks inside the unit.
- Training and education—multimedia tutorials.

Originally there were just washers, not washer disinfectors. Don’t get caught out. There are chemical washers that use high strength chemicals to disinfect. There are thermal disinfectors that use high temperature water (90°C) to achieve disinfection. Review the Instructions for Use (IFU’s) as some may state one over the other.
Thermal WD are generally preferred as they are less hazardous and more environmentally friendly as a result. What AO value does the disinfector achieve and does it monitor this?
Some washers have built in ultrasonic—can all your instruments go in this?
Chemicals (cleaning agents) in a thermal washer. The detergent is used to clean, removing debris and soil/protein. Neutraliser is used to bring the pH to balance ready for disinfection. No chemical is added for disinfection as this is done by temperature, not chemical. Then a few mls of rinse aid is added before drying to aid in drying efficiency.
Some washers do not have ‘active drying’ which is fan forced. Some don’t bring air through a HEPA filter and simply run surrounding air through the once highly disinfected instruments—caution!
Be aware that most hollowed instruments will still need blowing out with compressed air.
Possible future proofing—De-ionised / de-mineralised water. Depending on the standards update, it may be a requirement to rinse with demineralised water. Does the washer have an option to add a water treatment system if standards change? If so, what is the cost of the add on?
All in all, the best practice reprocessing management is to – Make it Mechanical!
—
This article originally appeared in Volume 216, December 2023 of NZDA News.



 Coaching Solutions
Coaching Solutions













